Data presented from the ALIGN-AR Trial
The Trilogy THV System met prespecified non-inferiority endpoints for both safety and efficacy in high-risk patients with symptomatic, severe aortic regurgitation.
Watch the PresentationPrimary 30 day safety endpoint* met
The Trilogy valve met a prespecified non-inferiority performance goal based on historical data (p < 0.0001).
*Composite of 30 day all-cause mortality, all stroke, life-threatening/major bleeding, major vascular complications, AKI ≥ 2 or dialysis, valve intervention, new permanent pacemaker, ≥ moderate PVR
| Variable | % (n) |
|---|---|
| All Cause Mortality | 2.2% (4) |
| Cardiovascular Mortality | 2.2% (4) |
Any Stroke
|
2.2% (4) 1.1% (2) 1.1% (2) |
| Major/Life Threatening Bleeding | 4.4% (8) |
| Major Vascular Complication | 3.9% (7) |
| Acute Kidney Injury Stage 2 or 3 or Dialysis (7 Days) | 1.1% (2) |
| Surgery/Intervention Related to the Device | 2.8% (5) |
New Pacemaker Implantation
|
24.0% (36) 16.7% (30) |
| ≥ Moderate Paravalvular Regurgitation | 0.6% (1) |
| Total | 26.7% (48) |
Lower rates of new pacemaker observed toward the end of the trial
New pacemaker rates decreased with changes to procedural technique, sizing, and management of periprocedural conduction abnormalities.
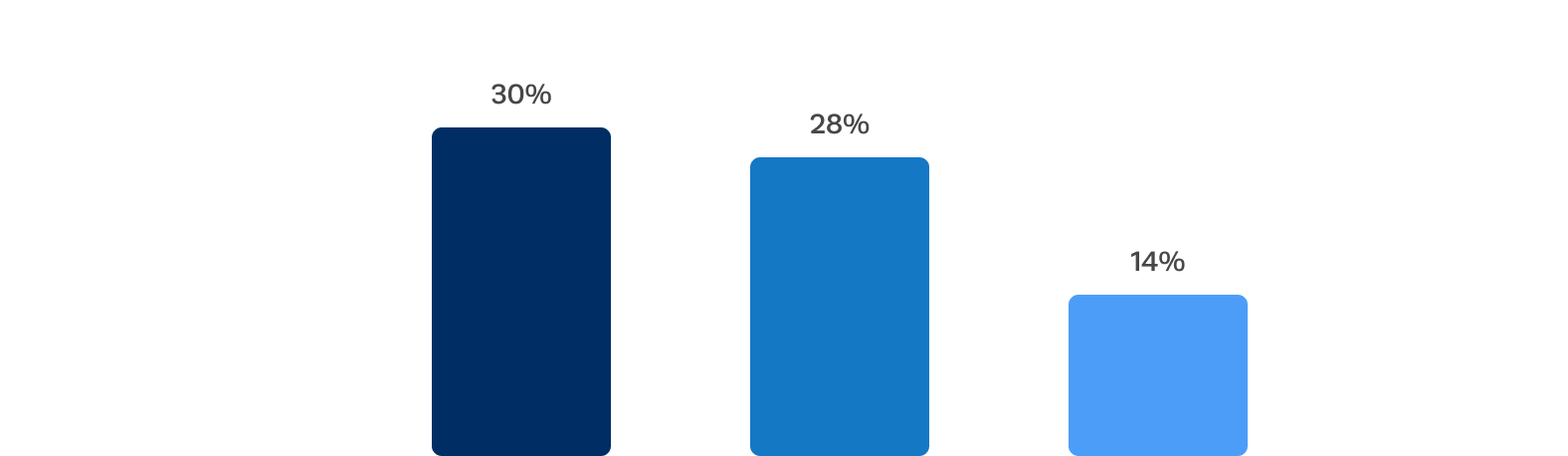

Primary 1 year efficacy endpoint* met
Untreated severe, symptomatic aortic regurgitation is associated with high mortality. The Trilogy valve met a prespecified non-inferiority performance goal comparing treatment with Trilogy TAVR to conservative management (p < 0.0001).
*All-cause mortality
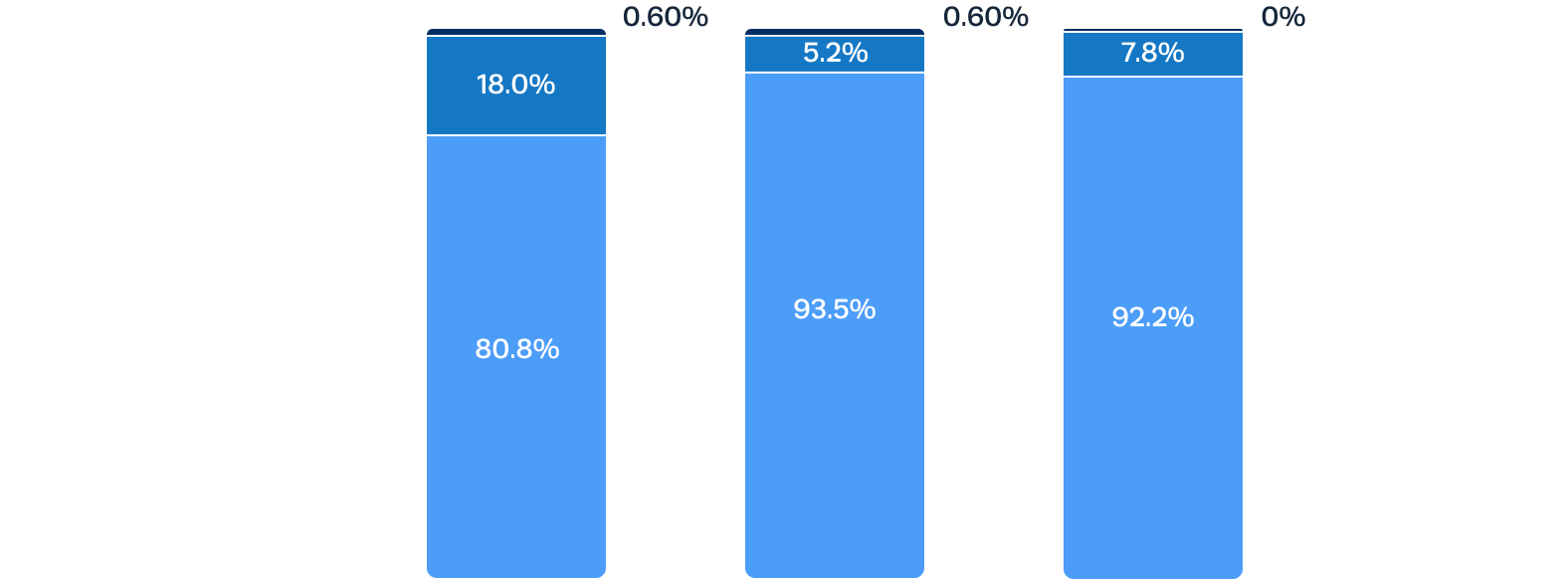
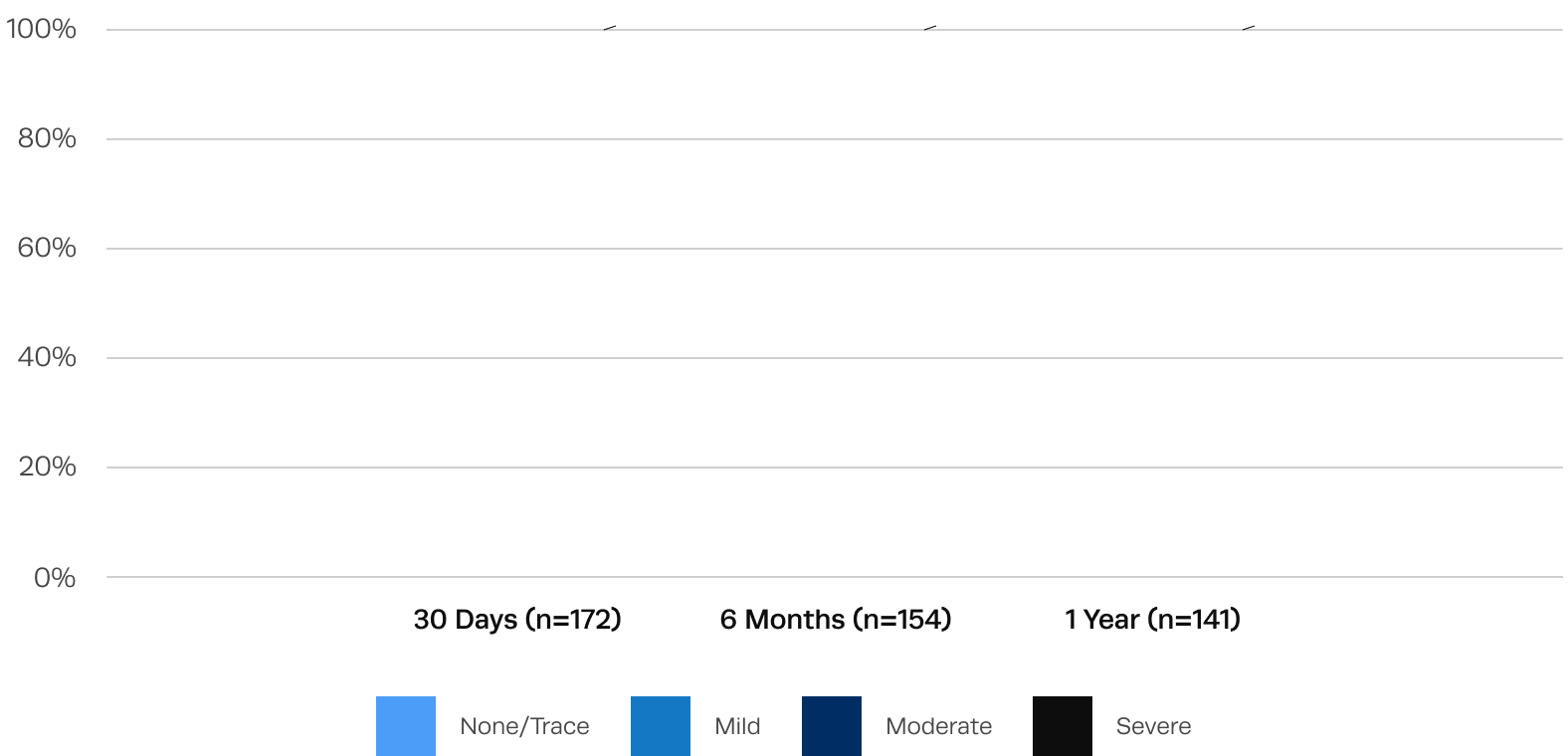
0% moderate and only 7.8% mild paravalvular regurgitation at 1 year after Trilogy TAVR
Residual aortic regurgitation is a common complication of off-label use of TAVR devices to treat AR.1 After Trilogy TAVR, significant aortic regurgitation was eliminated in all patients.
1. Haddad A et al., Clinical Cardiology 2019;42:159-166.
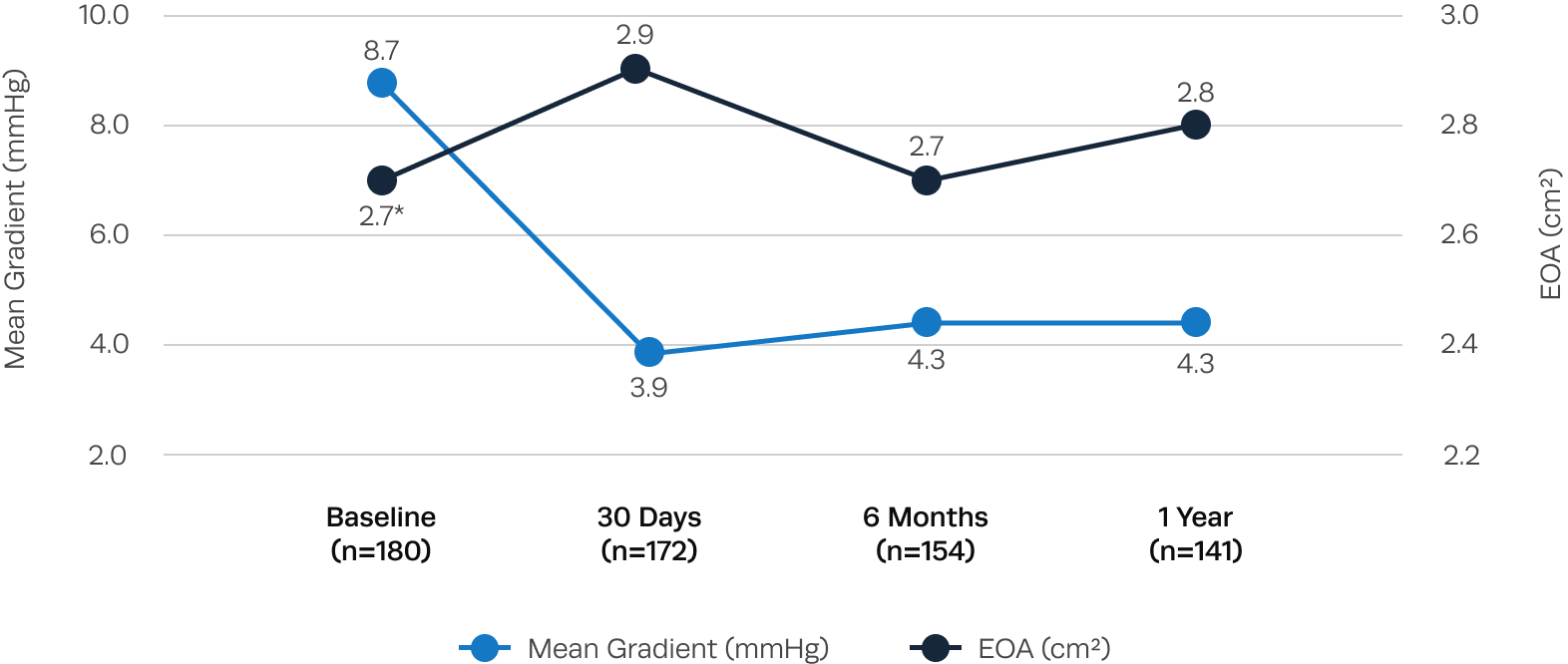
Excellent hemodynamic valve performance
Low single-digit transvalvular gradients and large EOAs observed after Trilogy TAVR.
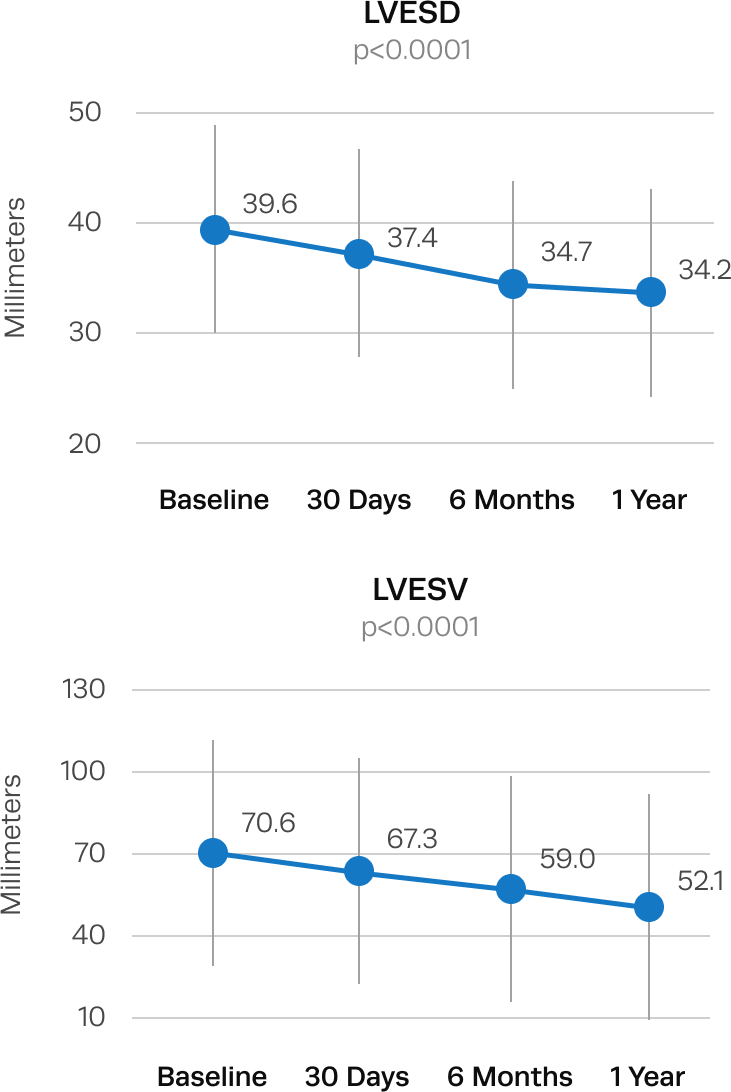
Significant left ventricular remodeling and reduction in LV mass after Trilogy TAVR
Left ventricular dilation is seen in patients with aortic regurgitation. After Trilogy TAVR, patients experienced significant improvements in LV remodeling.
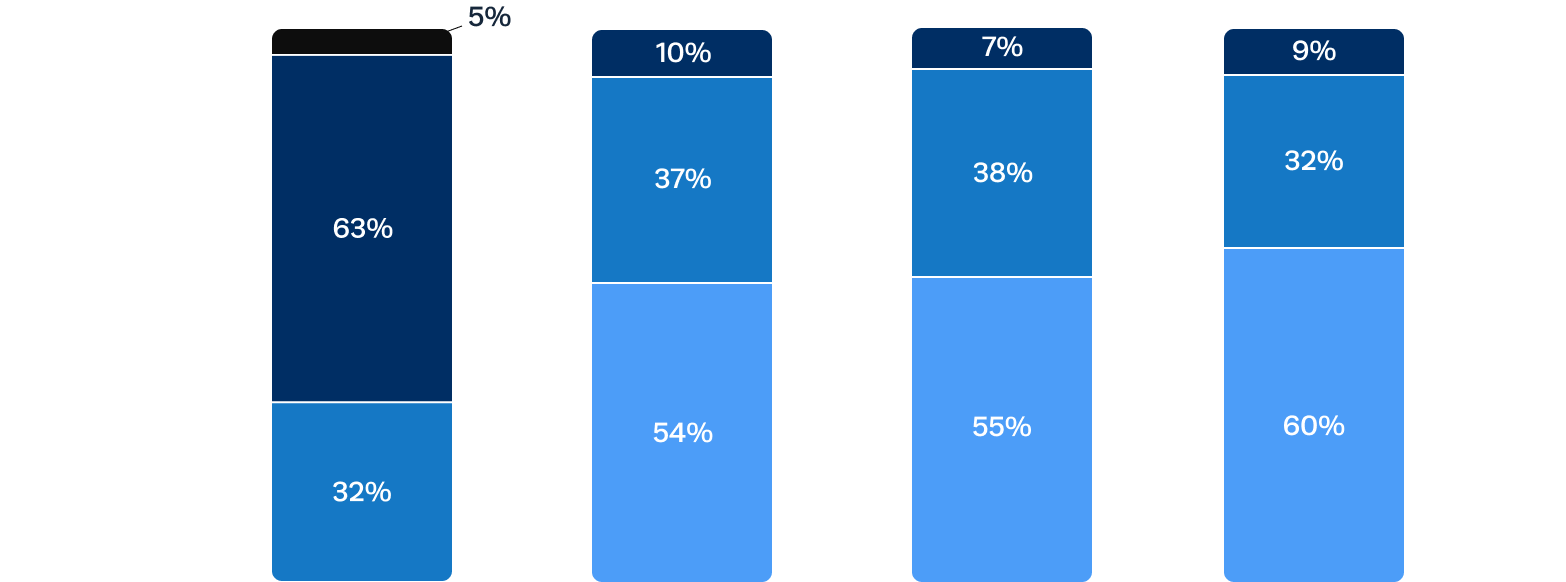
Sustained improvements in quality of life after Trilogy TAVR
At 1 year, patients experienced improvements in functional status with 92% in NYHA I/II, compared to 68% of patients in NYHA III/IV at baseline.